
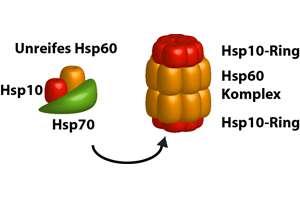
Discussedīelow are a few of the most common classes of molecular chaperones and their effects on protein folding in the cell. The activity of chaperones often requires the binding and hydrolysis of adenosine triphosphate (ATP).Īlthough only 20 to 30 percent of polypeptide chains require the assistance of a chaperone for correct folding under normal growth conditions, molecular chaperones are absolutely required for cell viability. Chaperones promote correct folding of their substrate proteins by unfolding incorrect polypeptide chain conformations, and, in some cases, by providing a sequestered environment in which correct protein folding can occur.
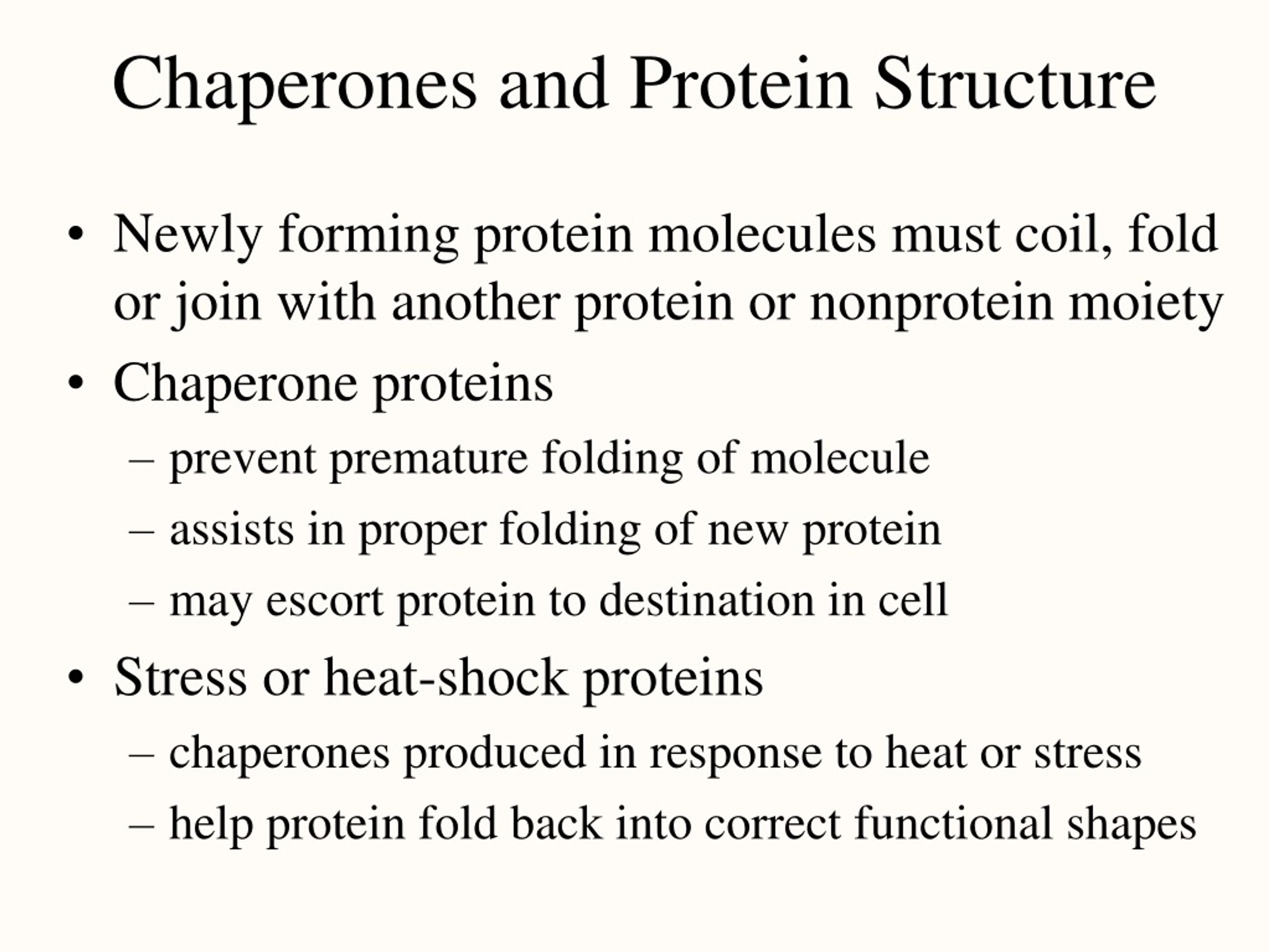
In correctly folded proteins, these surfaces are usually buried away from the watery environment surrounding the protein. Recognizing and Correcting MistakesĬareful study, both in vivo and in the test tube, has demonstrated that molecular chaperones bind to their non-native substrate proteins by recognizing exposed non-polar surfaces ("non-polar" means that they are not attracted to water). Most chaperones are also abundantly expressed under normal cell growth conditions, where they recognize non-native conformations occurring during both protein synthesis (prior to correct polypeptide chain folding), and later misfolding events. It was later discovered that chaperones recognize the non-native, partially misfolded states of proteins that accumulate during high temperature stress. It was quickly determined that this "chaperone" protein directing correct assembly was identical to one of the many proteins expressed at high levels when cells are grown at high temperatures (hence the common alternative name, "heat-shock protein," or Hsp). A new protein was identified that was required for correct folding of a large enzyme complex in chloroplasts, yet the mysterious protein was not associated with the final assembled complex. Discovery of ChaperonesĬhaperones were originally identified in the mid-1980s from studies of protein folding and assembly in plant chloroplasts. Chaperones are found in all types of cells and cellular compartments, and have a wide range of binding specificities and functional roles. The misfolded or unfolded polypeptide chains to which chaperones bind are said to be "non-native," meaning that they are not folded into their functional conformation. All proteins are created at the ribosome as straight chains of amino acids, but must be folded into a precise, three-dimensional shape (conformation) in order to perform their specific functions. Molecular chaperones are proteins and protein complexes that bind to misfolded or unfolded polypeptide chains and affect the subsequent folding processes of these chains.


 0 kommentar(er)
0 kommentar(er)
